Regarding Ribosomes with Venki Ramakrishnan, PhD
This article was written in celebration of the opening of a new interactive at NHMU inspired by Dr. Venki Ramakrishnan's award-winning research on the ribosome. See it in the Life Exhibition.
By Michael Mozdy
Our body is riddled with ribosomes – every single cell contains thousands of these protein-making factories. It’s a good thing, too, because without them we wouldn’t be alive.
“Everything in the cell is either made by the ribosome or made by enzymes that are themselves made by the ribosome,” states Venki Ramakrishnan, PhD, who shared the 2009 Nobel Prize in Chemistry with Thomas A. Steitz and Ada Yonath for research on the structure and function of ribosomes.
DNA and RNA are widely famous for being the secret code of life, but “without ribosomes you just have a blueprint,” asserts Ramakrishnan. Every living creature relies on ribosomes to “read” DNA’s genetic instructions and make proteins and enzymes that do the work of life.
The process from DNA to protein has three main steps:
- Enzymes “transcribe” sections of DNA containing genetic information onto a long strand of RNA called messenger RNA (mRNA). Think of mRNA as the middle man.
- Ribosomes latch onto the mRNA and read its blueprint, forming amino acids for each link in the long chain of mRNA.
- The amino acid chain folds upon itself in complex ways to make a protein.
What’s the big deal about proteins? Well, they are involved in virtually everything that happens in a cell. They control cell division, metabolism, and the flow of materials and information into and out of the cell.
So, we see that ribosomes are the key piece of the puzzle: they take the genetic blueprint and make the main actors of the biological world, the proteins.
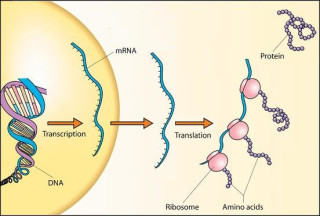
Image ©Noor Dawany
But … How?
From ribosomes’ discovery in 1955 until the end of the century, biologists knew that they were a big deal, but they couldn’t see any details of the ribosome itself.
“Think of being a Martian out in space and looking down on earth,” Ramakrishnan elaborates. “You’d see all these small objects moving around in straight lines. Then you notice that even smaller objects would enter these objects; and the only time these objects moved was when the little objects got into them. But you wouldn’t know anything about what was going on.”
Continuing the Martian observer analogy, Ramakrishnan says that over time you might note that the objects emit carbon dioxide and water and use up gasoline. “So, while you have some useful observations, unless you get to see that it has an engine and a steering wheel and a crankshaft, you would have no idea how it worked.”
This was the problem that Ramakrishnan set out to solve. By the 1990s, scientists knew that the ribosome had two parts, unimaginatively named the large subunit and the small subunit. They also knew that a number of things happened when it made proteins: the two subunits came together and other proteins and enzymes were recruited to the ribosome to help in the process. But, like Martians looking down on earth, they simply didn’t have a detailed enough picture to know how all of these things happened.
By the mid-1990s, an Israeli researcher named Ada Yonath had been working on the large subunit for a decade or more, but the work had yielded a stubbornly fuzzy picture of the structure. A Russian team of researchers had recently unveiled a similar level of characterization for the small subunit and the entire ribosome.
Ramakrishnan decided that a new emerging technology could be used to surpass the other efforts around the world. He could quite possibly get enough data and resolution with this method to peer all the way down to the atomic level and reconstruct a model of the ribosome. Rather than go head-to-head with Yonath on the large subunit, Ramakrishnan turned his sights on the small subunit and began the steps that he knew would take several years to work.
Little did he know that Yonath and others had also shifted to the small subunit. The race was on.
A Fascinating Career with a Stop in Utah
Ramakrishnan took an unconventional path to his discovery, and really his whole career. He received an undergraduate degree in physics in India, where he was born and raised. He then travelled to the United States to attend Ohio University where he received his PhD in physics. But his chosen field was a tough nut to crack.
“Questions in theoretical physics are quite hard,” he says. “It’s a mature field, dating back to Galileo 500 years ago.” During this time, he regularly read Scientific American and kept seeing exciting breakthroughs in biology. “Modern biology is just around 150 years old, and there is a lot more opportunity.”
He was heartened by the fact that a few well-known scientists like Francis Crick had successfully transitioned from physics to biology. So, he jumped fields.
He credits the support of his wife, Vera, with helping through the move back to graduate school at UCSD on a modest stipend when they had two young children. After two years, he decided he wanted to work on this cellular factory called the ribosome, so in 1978 he moved coast-to-coast to pursue a postdoctoral fellowship at Yale University in Chemistry.
Next, he spent 12 years in Brookhaven National Laboratory before taking a faculty position at the University of Utah in 1995. This was the point in time when he pursued the course of research that would eventually result in the Nobel Prize. Even then, however, Ramakrishnan wasn’t done with life upheavals. The life of a researcher at academic research universities in the USA is one where grant funding must be constantly and rigorously pursued. He considered how the ribosome had remained unyielding in terms of actionable data for decades and knew that continuing to receive grant money from the National Institutes of Health would be challenging. They prefer to fund projects that have momentum and a high chance of success.
Thus, when he had the opportunity to go to the MRC Laboratory of Molecular Biology in England that received central funding without the need for constantly jockeying and competing for grant money, he took it. He considered this more important than the pay cut he would take, and he and his wife left their grown children in the US to move to Cambridge, England in 1999.
Ramakrishnan believes that his path contains some important lessons for budding scientists: “If you find things don’t work out, you have to be open to change.” He lived this by example, changing fields from physics to biology when the opportunity presented itself, moving from Utah to England when funding was more guaranteed, and taking a pay cut in order to complete his investigations.
The Prize
In Ramakrishnan’s mind, the prize was never the Nobel – that was a happy and unforeseen result – but rather the atomic structure of the ribosome. Ironically, by the time he moved to England, his lab in Utah had already made their key initial breakthrough. In August 1999, they published their important paper “Structure of a bacterial 30S ribosomal subunit at 5.5 A resolution.” in the journal Nature.
A year after they moved to Cambridge, they published the complete structure of the small subunit in 2000, also in Nature, shortly after Thomas Steitz, Peter Moore, and their colleagues from Yale University published the structure of the large ribosomal subunit in the journal Science. Yonath also published her group’s structure of the small subunit in the journal Cell.

Ramakrishnan with members of his lab on the day his Nobel prize was announced in 2009. ©MRC Laboratory of Molecular Biology
The early 2000s saw a flurry of exciting publications from all three groups. It was a watershed moment for the ribosome. In 2006, an entire functional ribosomal complex was unveiled in high resolution, and biologists finally could see where exactly mRNA fit, how other proteins attached, and where the amino acid chain exited from the ribosome.
Ramakrishnan relates the excitement and twists of this classic scientific race in his book, Gene Machine.
Venki Ramakrishnan is a truly inspiring and articulate scientist, one we are honored to feature in a new ribosome exhibit interactive at the Natural History Museum of Utah. Plan your visit today to come and dive into the microscopic world inside a cell and help a ribosome make a protein.




