Psychoactive psilocybin’s evolution in ‘magic mushrooms’
This article originally appeared on the University of Utah's @theU.
By Lisa Potter
Psilocybe fungi, known colloquially as “magic mushrooms,” have held deep significance in Indigenous cultures of Mesoamerica for centuries. They captured the wider world’s attention as a psychedelic staple in the 60s and 70s. Now, these infamous organisms are at the forefront of a mental health revolution. Psilocybin and psilocin, the psychoactive compounds found in nearly all species of Psilocybe, have shown promise as a treatment for conditions including PTSD, depression, and for easing end-of-life care.
To utilize psilocybin as a therapeutic, scientists need an extensive roadmap of the compound’s underlying genetics and evolution, information that doesn’t exist. Our limited knowledge comes from research on just a fraction of the ~165 known species of Psilocybe. Most psilocybin-producing mushrooms haven’t been studied since they were first discovered—until now.

Bryn Dentinger holding a collection of Psilocybe caerulescens, found performing field work in Jalisco, Mexico.
Photo by Bryn Dentinger
A team of researchers led by the University of Utah and the Natural History Museum of Utah (NHMU) has completed the largest genomic diversity study for the genus Psilocybe. Their genomic analysis of 52 Psilocybe specimens includes 39 species that have never been sequenced.
The authors found that Psilocybe arose much earlier than previously thought—about 65 million years ago, right around when the dinosaur-killing asteroid caused a mass extinction event. They established that psilocybin was first synthesized in mushrooms in the genus Psilocybe, with four to five possible horizontal gene transfers to other mushrooms from 40 up to 9 million years ago.
Their analysis revealed two distinct gene orders within the gene cluster that produces psilocybin. The two gene patterns correspond to an ancient split in the genus, suggesting two independent acquisitions of psilocybin in its evolutionary history. The study is the first to reveal such a strong evolutionary pattern within the gene sequences underpinning the psychoactive proteins synthesis.
“If psilocybin does turn out to be this kind of wonder drug, there’s going to be a need to develop therapeutics to improve its efficacy. What if it already exists in nature?” said Bryn Dentinger, curator of mycology at NHMU and senior author of the study. “There’s a wealth of diversity of these compounds out there. To understand where they are and how they’re made, we need to do this kind of molecular work to use biodiversity to our advantage.”

Alexander Bradshaw holds a specimen of Psilocybe zapotecorum collected while hunting for mushrooms near Talpa de Allende, Jalisco, Mexico.
Photo by Bryn Dentinger
All the study’s Psilocybe DNA came from specimens in museum collections around the world. Twenty-three of the 52 specimens were “type specimens,” the gold standard designating a species against which all other samples are measured. For example, say you identify a wild mushroom as a certain species of chanterelle—you’re betting that the mushroom you picked is the same as the physical material sitting in a box in a museum. The authors’ molecular work on type species is a major contribution to mycology because it establishes an authoritative foundation for all future work on Psilocybe diversity in taxonomy.
“These type specimens represent hundreds of years of thousands of scientists’ collective effort to document diversity, way before people were thinking about DNA,” said Alexander Bradshaw, postdoctoral researcher at the U and lead author of the study. “That’s the beauty of it—no one has really sequenced type specimens at this scale, and now we get to produce molecular and genomic data to the gold standard of Psilocybe types for people to compare against.”
The study published in the journal Proceedings of the National Academy of Sciences on Jan. 9, 2024.
A trip through time
Previous studies identified the cluster of four core genes that produce psilocybin based on genomic analysis of threePsilocybe species. The species were closely related to each other, and all had matching gene patterns within the psilocybin-producing gene clusters. This study’s expanded genomics of 52 specimens of Psilocybe revealed a second distinct pattern.
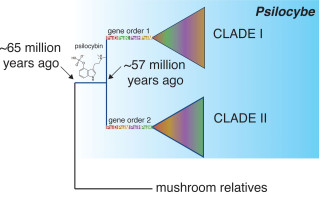
A simplified evolutionary tree for the genus Psilocybe. Clade I and Clade II represent the Psilocybe species with the same gene order patterns within their psilocybin-producing gene clusters.
Illustration by Bryn Dentinger
“This work represents a big step in the understanding of the evolutionary relationships in Psilocybebecause it is the first to include a broad species sampling and is based on type specimens,” said Virginia Ramírez-Cruz, mycologist at the Universidad de Guadalajara and co-lead author of the study.
The authors found that 17 specimens had the original order, while 35 exhibited the new pattern.
“We’ve shown here that there’s been a lot of change in gene order over time, and that provides some new tools for biotechnology. If you’re looking for a way to express the genes to produce the psilocybin and related compounds, you no longer have to rely on only one set of gene sequences to do that. Now there’s tremendous diversity that scientists can look at for lots of different properties or efficiencies,” said Dentinger, who is also an associate professor of biology at the University of Utah.
Dating of the group showed that an ancient split of the two gene cluster patterns occurred around 57 million years ago, which also corresponded to a shift in the ecology. The first psilocybin-producing mushrooms likely arose as a wood-decomposing group, then transitioned to soil after the split, with some species such as Psilocybe cubensis transiting to growing on herbivore dung. The ecological shift to dung appears to have occurred at least twice independently in their evolutionary history.
What does psilocybin do for mushrooms?
The authors hoped that psilocybin’s evolutionary history would clarify the most basic question—what does psilocybin do for mushrooms? The psilocybin-producing gene clusters likely have some benefit, but no one knows what it is.
The molecular structure of psilocybin mimics serotonin and binds tightly to serotonin receptors, especially at 5-HT2A, a famous receptor onto which many psychedelic drugs bind. When a chemical binds to these receptors in mammals and similar ones in insects and arachnids, they produce unnatural and altered behaviors. Some have proposed that this altered mental state might be a direct deterrent to predation. It’s also possible that psilocybin functions as a laxative or induces vomiting to spread spores before they are fully digested. However, psilocybin mushrooms often occur infrequently in the wild, making it unlikely that animals could learn to recognize them. An alternative theory is that psilocybin is a chemical defense against insects. However, empirical studies are lacking, and the authors’ personal observations confirm that psilocybin-containing mushrooms regularly host healthy, thriving insect larvae.

Holotype specimen of Psilocybe subtropicalis.
Photo by Eliza Petersen/NHMU
The authors are preparing experiments to test an alternative theory that they call the Gastropod Hypothesis. The timing and divergence dates of Psilocybe coincide with the KPg boundary, the geological marker of the asteroid that threw Earth into a brutal, prolonged winter and killed 80% of all life. Two lifeforms that thrived during the darkness and decay were fungi and terrestrial gastropods. Evidence, including the fossil record, shows that gastropods had a massive diversification and proliferation just after the asteroid hit, and it’s known that terrestrial slugs are heavy predators of mushrooms. With the study’s molecular dating of Psilocybe to around 65 million years ago, it’s possible that psilocybin evolved as a slug deterrent. They hope that their feeding experiments will shed some light on their hypothesis.
In 2020, the authors set a goal to get a genome sequence for every Psilocybe type specimen. To date, they’ve generated genomes of 71 type specimens and continue to collaborate with collections around the world.
“It’s impossible to overstate the importance of collections for doing studies like this. We are standing on the shoulders of giants, who spent thousands of people-power hours to create these collections, so that I can write an email and request access to rare specimens, many of which have only ever been collected once, and may never be collected again,” said Bradshaw.
Other authors who contributed to the study include Ali Awan of Guy’s and St. Thomas’ NHS Trust, Giuliana Furci of the Fungi Foundation, and Laura Guzmán-Dávalos of the Universidad de Guadalajara. The research was funded by the National Science Foundation (DEB #2114785) and Fungi Perfecti LLC.
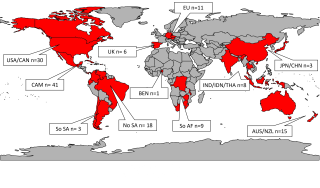
Institutions worldwide sent Psilocybe mushroom specimens in their collections to the U mycologist. Some had been collected and cataloged over 150 years ago. This figure lists specimens included in the study and new specimens being used for future studies as the authors continue to do genomic analysis on more Psilocybe species.
Illustration by Alexander Bradshaw



